Paneer 101
A deep dive into the Indian Cuisine superstar cheese!
Whenever I make paneer at home, I’m always amazed at how a lump of cheese seems to magically emerge from a smooth liquid like milk. Think about it—chemically, it’s easy to imagine dissolving sugar in water and reversing it by boiling it back down to sugar crystals. But can you imagine dissolving paneer solids in water and expecting it to turn back into milk? It is kind of a 1-way process, isn’t it?
It’s incredible how mammals can suspend crumbly proteins, fats, sugars, and minerals in water to deliver something as smooth and unified as milk. The chemistry of milk is astonishing—and once you understand it, you can make some truly amazing things with it.


Chemistry of Paneer
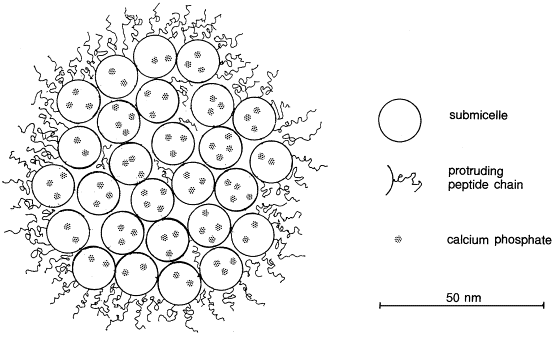
Milk contains several proteins, with casein being the most abundant. Proteins are long chains of amino acids—much larger than typical molecules, though still invisible to the naked eye. In milk, casein molecules are bundled into structures called micelles, which are like tightly wound balls of yarn. Inside, alpha and beta casein proteins are packed tightly, while kappa casein forms hair-like structures on the outer surface. Normally, these micelles repel each other while being attracted to water, which keeps them evenly suspended and gives milk its uniform appearance.
When you add acids like lemon juice or vinegar to milk with the addition of heat, all hell breaks loose. Acids have tons of positively charged protons. When acids are added, the protons act like a bull set loose in a china shop [or a horse in a hospital:)] messing with the system denaturing and coagulating some of the proteins, especially those on the surface level. They release hydrogen ions into the liquid. The more acidic the substance, the more hydrogen ions it contributes. The heating of milk further accelerates the reaction.
At lower acid levels, similar to what occurs during fermentation, milk proteins form soft gels—like yogurt. This takes place over several hours and it is a gentle process. But in the case of paneer, we are not doing this low and slow. We are hitting the milk with intense acidity and heat- Bam! As the acidity increases, the micelles lose their charge and stop repelling each other. With no charge and no water attraction, they clump together and expel water—forming paneer.



Buffalo vs Cow’s milk Paneer
Buffalo milk is generally preferred over cow milk for making high-quality paneer. Buffalo milk paneer is more soft, spongy and porous when compared to cow's milk paneer. Buffalo milk has a fat content of 7–8%, which is almost double that of cow's milk, which has 3–4% fat. Fat content matters. The fat globules hinder the fusion between protein strands preventing them from forming stronger bonds with the surrounding proteins. Higher fat equals softer mouthfeel.


Cow milk, on the other hand, is preferred for making chenna—the base for sweets like rasgulla and chum chum. It produces a better result ideal for these desserts. Chenna made from buffalo milk often turns out coarse, greasy, and too firm, which isn’t great for high-quality sweets.

One common complaint about store-bought paneer (be it buffalo or cow) is that it's often too hard. When you make it at home, it’s fresher, softer, and ready to use right away. However, commercial producers have to reduce moisture to extend shelf life—just a 5–10% reduction which makes a big difference. They also use vacuum packing and refrigeration. They need to use all means possible on their end to reduce microbial contamination. As a consumer, you are able to pick up that drop in packed moisture when you chew on it. In paneer, once the moisture is squeezed out, it is hard to get it back inside (will discuss more in the next post). Moisture content and fat content matters a whole lot!
But even home made paneer can run into problems. I will discuss my experiments making paneer and show you the effects of changing variables (like diff acids, which temperature, fat content, etc) in the next post. —some of the results might surprise you! I hope this basic beginner post helps you appreciate paneer more. Happy cooking!


Simple and informative, this post taught me a lot!
Waiting for your next posts, just subscribed. You broke it down quite interestingly yet in a crisp manner, thanks for sharing.